
The electron cloud model uses the concept of ‘orbitals’, referring to regions in the extra-nuclear space of an atom where electrons are likely to be found. Based on quantum mechanics, it gives the probable location of electrons represented by an ‘electron cloud’. The model does not depict electrons as particles moving around the nucleus in a fixed orbit. The electron cloud is also defined as the region where an electron forms a three-dimensional standing wave, the one that does not move relative to the atomic nucleus.
The model is used to describe the probable locations of electrons around the atomic nucleus. It is a visual model that maps the possible locations of electrons in an atom. The model provides the means of visualizing the position of electrons in an atom. On similar lines, an electron can fall to a lower energy level by emitting a photon, thus radiating energy. On absorbing a photon, an electron moves to a new quantum state by acquiring a higher level of energy. Each orbital is equivalent to an energy level of the electron. Where in the atom are electrons found?Įlectrons orbit around the nucleus of an atom. Hence the energy required to separate an electron from the atom varies inversely with its distance from the nucleus. The attractive force of an electron is directly proportional to its distance from the atomic nucleus. It’s an electromagnetic force a force of attraction that exists between the electrons and the nuclear protons, and binds them to the atom. What binds the subatomic particles in an atom together? You might also like to know about the parts of an atom before exploring the concept of an electron cloud.
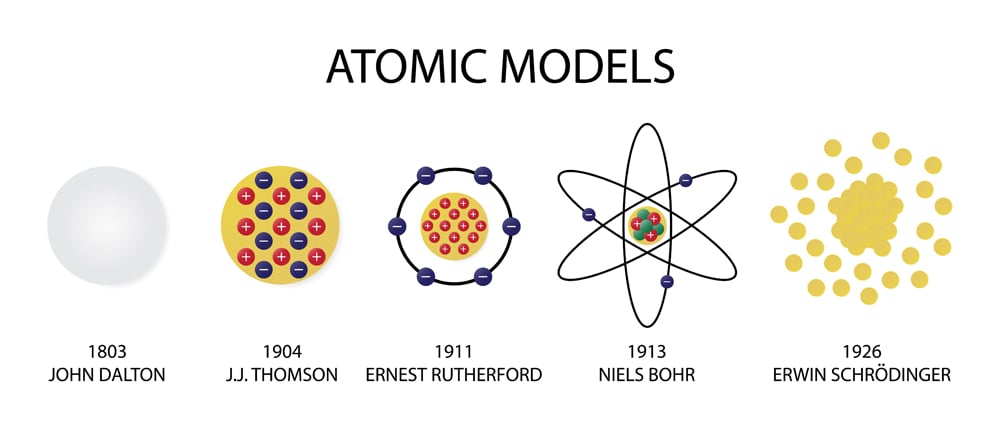
The electron cloud refers to a region outside the nucleus where an electron is most likely to be found.īefore understanding what an electron cloud model is, it is important to know about the forces that bind the electrons together. Quick ReadĮrwin Schrodinger, an Austrian physicist came up with the electron cloud model in 1926. What is an electron cloud model? Who proposed the concept of an electron cloud? Read on to find out.


 0 kommentar(er)
0 kommentar(er)
